Abstract
Background: High dose melphalan and autologous stem cell transplant (SCT) in first remission, followed by lenalidomide maintenance, improves progression-free survival (PFS) but is generally not curative. Achieving a minimal residual disease (MRD)-negative state at 12 months post-SCT, particularly at the greatest depth of sensitivity (i.e. 10-6), is one of the strongest predictors of prolonged PFS and overall survival (OS). Belantamab mafodotin (belamaf) is a novel BCMA-targeted antibody-drug conjugate approved for relapsed/refractory myeloma. We hypothesized that incorporating belamaf with autologous stem cell transplant and lenalidomide maintenance would improve MRD-negative rates and long-term clinical outcomes. We also hypothesized that giving belamaf at a reduced frequency (every 3 months) would reduce incidence and severity of ocular adverse events and improve tolerability.
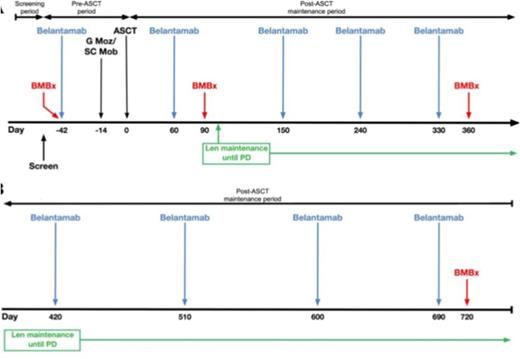
Study Design and Methods: This is an ongoing investigator-initiated, single institution, single arm phase 2 study (NCT04680468) that opened June 2021, with plans to enroll 39 evaluable subjects. Funding support is provided by GSK. Subjects must be within 12 months of initiating therapy for myeloma and have achieved at least a partial response (PR) after 4 or more cycles of induction therapy (choice of induction not specified by study), but cannot be in a stringent complete response (CR) post-induction, and cannot have prior progressive disease. They must meet standard institutional criteria for autologous SCT with melphalan 200 mg/m2 conditioning. Subjects with active corneal epithelial disease are excluded. The trial schema is shown in the Figure. Subjects receive 1 dose of belamaf 2.5 mg/kg IV ~4 weeks before stem cell mobilization and collection with G-CSF and plerixafor and ~6 weeks before undergoing standard of care autologous SCT. They then restart belamaf starting day +60 post-SCT, continuing every 3 months up to 2 years post-SCT, in conjunction with standard lenalidomide maintenance (10mg/day for 21/28 days starting around day +100) given until progression. Subjects undergo serial ophthalmologic exams, with belamaf dose modifications and/or holds for corneal toxicity based on the Keratopathy and Visual Acuity (KVA) scale, as used in other belamaf trials. Other toxicities are graded by NCI CTCAE version 5.0. Responses are assessed by IMWG criteria. MRD is assessed on bone marrow aspirates at enrollment and 3, 12 and 24 months post-SCT by next generation sequencing (NGS, Adaptive Clonoseq assay). Health-related quality of life (HRQoL) is assessed by FACT-MM questionnaire. Correlative analyses include serial assessments of soluble BCMA in serum as well as BCMA expression on residual myeloma cells (if present) by flow cytometry.
The primary endpoint is the rate of MRD-negativity as assessed by NGS with a sensitivity of at least 10-6 at 12 months post-SCT. The null hypothesis is that the true MRD-negativity rate is 30% and the alternative hypothesis is that the true MRD-negativity rate is 50%. With 39 evaluable subjects, the null hypothesis will be rejected if 17 or more subjects are MRD-negative (at 10-6) at 12 months post-ASCT. This design has a one-sided type I error rate of 0.05, and 83% power for an exact binomial test, when the true MRD-negative rate is 50%. Secondary endpoints include feasibility, safety, and tolerability of belamaf administered in the pre- and post-SCT setting; efficacy as assessed by IMWG responses and Kaplan-Meier estimates of PFS and OS; rates of MRD-negativity at 3 and 24 months post-SCT, and impact of belamaf on stem cell collection and hematopoietic reconstitution post-SCT, as well as on HRQoL. Exploratory endpoints include the frequency of contaminating myeloma cells within mobilized stem cell products, as well as pre- and post-therapy expression of BCMA on residual marrow myeloma cells (if detectable) and serum concentration of soluble BCMA, and relationship of these factors to clinical outcome measures.
Conclusion: The BLAST study seeks to assess the efficacy, safety, and tolerability of reduced-frequency belamaf when given pre- and post-SCT in conjunction with lenalidomide maintenance, in an effort to improve 12-month post-SCT MRD negativity rates at the most stringent level of sensitivity, a pre-requisite to improving the fraction of patients achieving durable remission. Accrual is ongoing.
Disclosures
Cohen:Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Ichnos, Janssen Oncology, Oncopeptides, Pfizer, Seattle Genetics, Genentech/Roche, AstraZeneca, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline and Novartis: Research Funding; Novartis: Patents & Royalties: CAR T-cells and biomarkers of cytokine-release syndrome. Garfall:Novartis, Janssen, Tmunity, CRISPR Therapeutics: Research Funding; Janssen, GSK, Amgen, Legend: Consultancy. Vogl:Oncopeptides: Consultancy; Karyopharm: Consultancy; CSL/Behring: Consultancy; Sanofi/Genzyme: Consultancy; GSK: Consultancy; Takeda: Consultancy, Research Funding; Active Biotech: Research Funding. Stadtmauer:BMS, Celgene, Abbvie, Sorrento: Research Funding.
OffLabel Disclosure:
Belantamab mafodotin, use in conjunction with stem cell transplant and maintenance.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal